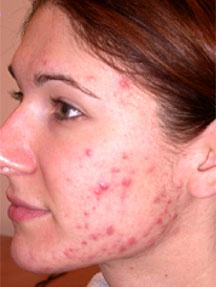
Ranbaxy Laboratories’ US based subsidiary Ranbaxy Laboratories Inc., (RLI) has received US FDA approval for Absorica, a novel, patented brand formulation of the acne medication isotretinoin, developed by Cipher, for the treatment of severe recalcitrant nodular acne. RLI has entered business agreement with Ciphar, the Canadian firm, to launch Absorica in the US market. It is expected that the product will be launch in fourth quarter of 2012 and RLI will pay royalties on net sales to Cipher Pharmaceuticals.